Group 17 Periodic Table
Lithium sodium and potassium for example all contain one valence electron in their atoms and belong to group 1 of the periodic table. Interactive periodic table of elements - your complete guide to the elements including definition mass names of each chemical in the periodic table.

Physical And Chemical Properties Of Group 17 Elements A Plus Topper Https Www Aplustopper Com Physical And Chemical Properties Chemical Property Chemical
The block names s p d and f are derived from the spectroscopic notation for the value of an electrons.

. Three systems have been used to number families and groups. The chemical elements of the same group had similar properties. As we have seen in post 28 the sequence of filling the orbitals is such that the energy of the ns shell is always less than n-1 d shell So the electrons fill the ns shell first and later they.
The above article covers all the important trends of properties in the periodic table. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. Interactive periodic table of the chemical elements.
18 Ar Argon 39948 2 8 8. It includes Fluorine F Chlorine Cl Bromine Br Iodine I and Astatine At. 1These elements are found in groups 3 to 12 in the periodic table.
- Group 18 VIIIA - 8 valence electrons. Mnemonic for Group 17. The Periodic Table provides the names atomic numbers symbols and atomic weights of known elements.
Halogen group is the group 17 on the periodic table. 2In these elements the penultimaten-1 d-orbital is filled with electrons. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
Recognizing Families on the Periodic Table. The periodic properties in the periodic table develop a base in order to understand the nature of elements in an efficient way. 20 Ca Calcium 40078 2 8 8 2.
Group 17 is known as the group of Halogens. Home About This Site Comments Help Links Window Version. Fir Call kar Bahaar AayI Aunty.
Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all. Skip to main content. The term appears to have been first used by Charles Janet.
22 Ti Titanium 47867. So elements from this group typically exhibit a -1 oxidation state. Each element in a periodic table group has the same number of valence electrons.
19 K Potassium 39098 2 8 8 1. Representative metals are members of s block and parts of p block of the table Elements of group 1 except hydrogen 2 13 except boron tin and lead of group 14 and bismuth of group 15 are. The Chemistry Divisions Periodic Table describes the history properties resources uses isotopes forms costs and other information for each element.
- Group 17 VIIA - 7 valence electrons. The elements of a Mendeleevs table were arranged in rows called periods and columns called groups. The elements included in halogen group are.
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. S-block p-block d-block and f-block. Alkali Metals Alkaline Earth Metals.
Columns of the periodic table typically mark groups or families. The alkali metals make up most of. As a result each groups valency is.
Properties history name origin facts applications isotopes electronic configuation crystal structure. Fluorine F Chlorine Cl Bromine Br Iodine I Astatine At Tennessine Ts For detailed information on halogens read the main article on Halogen elements of. Group 17 elements have a valency of 1 and group 18 elements have a valency of 0.
Each block is named after its characteristic orbital. This interactive periodic table of element groups arranges the chemical elements according to periodicity or common properties. There are different regions in the periodic table that are called periodic table blocks as they are named according to the subshell of the last electron of the atom.
26 Fe 5585. An up-to-date periodic table with detailed but easy to understand information. 17 Cl Chlorine 3545 2 8 7.
21 Sc Scandium 44956 2 8 9 2. Future Groups of the Periodic table Alkali metals. Name Atomic Number Atomic Mass Electron Configuration Number of Neutrons Melting Point Boiling Point Date of Discovery Crystal Structure.
While melting and boiling points of nonmetals increase on moving from top to bottom in a group of the periodic table. It serves as a great tool for solving chemistry problems. The periodic table of elements is arranged into several broad groups Image credit.

Tabel Periodik Kimia Bilangan Oksidasi
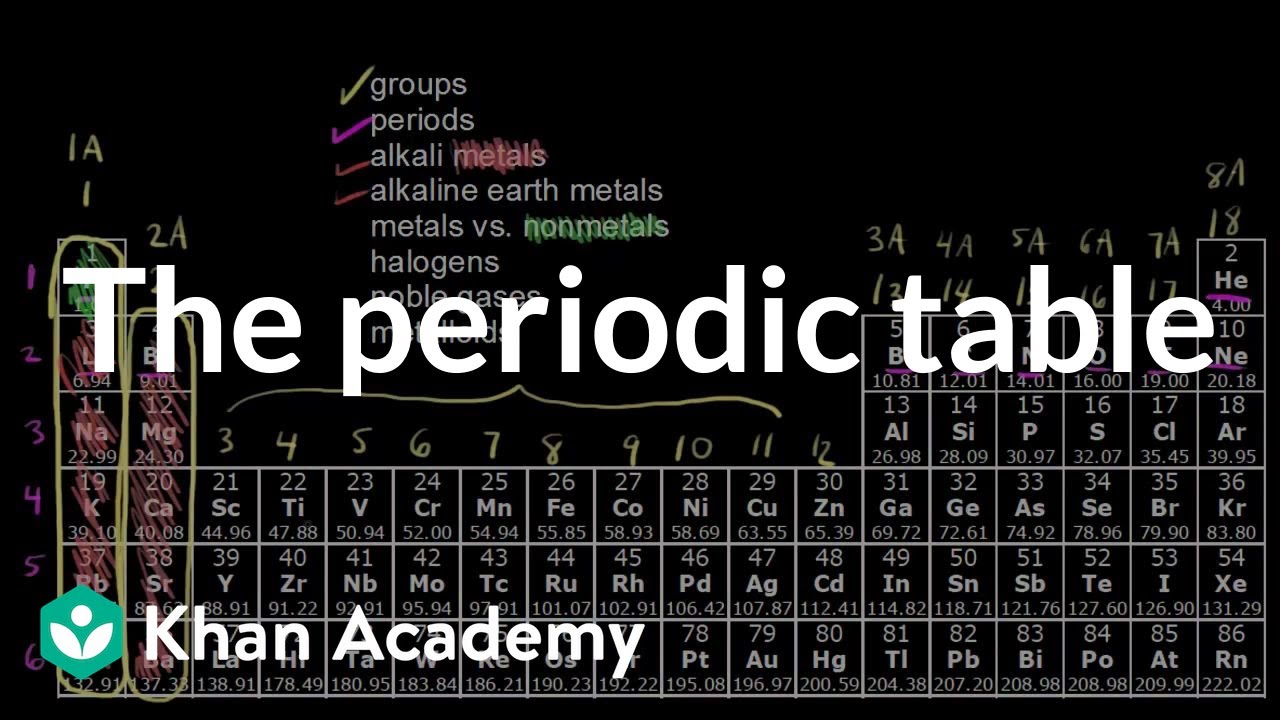
Definitions Of Groups Periods Alkali Metals Alkaline Earth Metals Halogens And Noble Gases How Metals Non Meta Periodic Table Chemistry Chemistry Basics

Periodic Table Of The Elements Chart Periodic Table Of The Elements Element Chart Teacher Created Resources

What Is The Periodic Table Of The Elements A Plus Topper Https Www Aplustopper Com His Periodic Table Periodic Table Of The Elements Relative Atomic Mass

Halogen Easy Science Periodic Table Halogen Easy Science

Actinoid Element Chemical Element Group Periodic Table Of The Elements Periodic Table Sms Language
0 Response to "Group 17 Periodic Table"
Post a Comment